
Prunus has passed the ISO13485 international quality system certification. All products have been awarded CE and CFDA(NMPA) certificates. Our products are exported to hundreds of countries and have entered the core departments of many of the world's top hospitals and research institutions. Prunus is an important OEM/ODM partner for dozens of top international brands for complete machines or components.
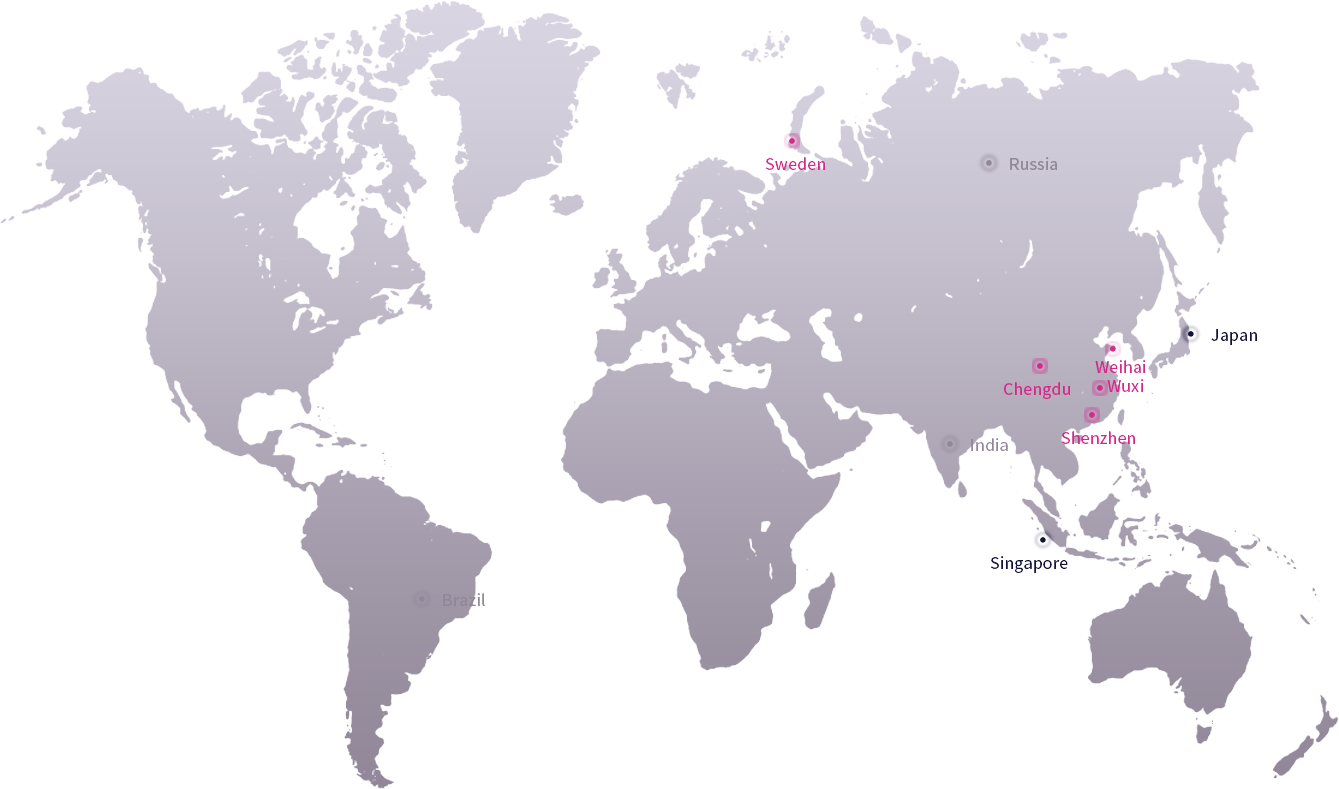














Prunus has always attached great importance to product research and innovation, maintaining a high level of investment in R&D and insisting on product innovation. With the rapid growth of its business, Prunus has established several R&D and manufacturing centers around the world. Working closely with more leading clinical experts and medical institutions worldwide through a combination of "industry, academia, research and medicine" keep us at the forefront of technological innovation.
Insisting on "Quality First", we have established a manufacturing center in accordance with international standards and established a rigorous and professional standardized production management process to guarantee the delivery of high quality products.

When the epidemic came, Prunus tried our best to protect lives. We were awarded the title of "Advanced Group of Industry and Information Technology System in Combating New Coronary Pneumonia Epidemic" in China and received unanimous recognition from many overseas countries.

Without forgetting the social responsibility, Prunus has been concerned more about public welfare, doing what it can for the meaningful public welfare of the society.